Some of you may have heard me quote a thoughtful essay by Daniel Shapiro and Kent Vrana (both of Pennsylvania State College of Medicine) that is critical of research institutions promoting what funds they’ve received over what scientific progress those funds have supported. The authors argue that instead of using a ranking system to measure success that favors number of grants and dollars, we should consider a new system that focuses on the efficiency by which the science was conducted and how the research contributes to answering questions that are meaningful to science.
With that in mind, it’s worth reflecting that it has been more than two years since the COVID-19 pandemic began. Let’s look back to the months of early to mid-2020 when the nation (and the rest of the world) faced a “novel” coronavirus, one which we knew could be fatal and for which there was little knowledge about how it spreads and no known effective treatment, limited diagnostic tests, and no vaccine. How did NIH make fast and meaningful contributions to respond to the pandemic?
On February 21, 2020, weeks before many of us were sent home from workspaces, the international Adaptive COVID-19 Treatment Trial (or “ACTT-1”) began enrolling patients into a definitive randomized trial on remdesivir in hospitalized patients. The trial was largely (though not completely) funded by NIAID and NCI through existing large-scale contracts and cooperative agreements. The trial stopped enrolling patients on April 19, 2020, only 1 month after we were sent home, and published a preliminary report of its main results on May 22, 2020. The trial, which enrolled 1,062 patients, showed positive results, meaning that remdesivir improved clinical outcomes safely. NIH-funded researchers leveraged extensive existing infrastructure to respond rapidly to the public health emergency and identify, in a definitive manner, an effective treatment within a matter of a few months.
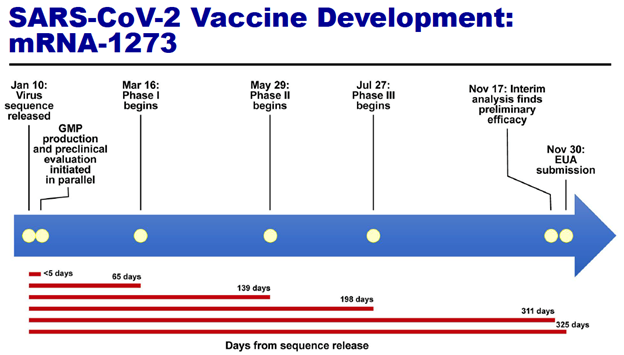
Also in early 2020, NIH intramural researchers as well as NIH-supported extramural researchers leveraged years – decades even – of research discoveries to develop and test a candidate COVID-19 vaccine. Figure 1 (taken from a December 10, 2020, presentation given by Dr. Anthony Fauci to the NIH Advisory Committee to the Director) shows the timeline of their efforts. A Phase 1 trial began within 65 days from when the virus sequence became known; the definitive Phase 3 trial, which enrolled over 30,000 volunteers, began 198 days after sequence release. The trial was led and supported by the HHS Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority and by NIAID through large-scale contracts and cooperative agreements. Like ACTT-1, the trial was positive, with the vaccine showing 94% efficacy for symptomatic COVID-19 infection and high levels of safety. The vaccine itself, based on an mRNA platform, was itself quite novel. The time from virus sequence to FDA Emergency Use Authorization submission was only 325 days.
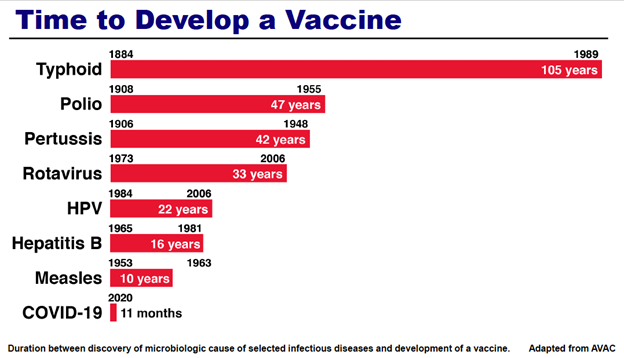
To put this in perspective, see Figure 2, also taken from Dr. Fauci’s presentation. The Figure shows the duration between the discovery of a microbiologic cause of disease and development of a vaccine. For typhoid, that was 105 years. Polio: 47 years. HPV: 22 years. Measles: 10 years. But for COVID-19 in 2020, only 11 months.
There were other important developments in early 2020 for which NIH and its partners leveraged resources, existing and new (e.g., through Congressional supplemental allocations), to respond to the pandemic. These included the establishment of the “Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)” program, described as “an unprecedented partnership for unprecedented times.” ACTIV projects have led to important discoveries about treatments that work and, equally important, treatments that do not work. Another was the Rapid Acceleration of Diagnostics (RADx®) initiative, which employed a shark tank-like approach and other approaches to support and stimulate development of diagnostic tests. In two years, companies supported by RADx have added approximately 2 billion tests and test products to the U.S. capacity.
I have only summarized a few of the events of 2020, but nonetheless some key themes emerge. First, NIH worked closely with partners in and out of government to leverage existing infrastructure and newly available funding to pivot rapidly (on a scale of days to weeks to months) in response to the pandemic. And second, these efforts (and many others) reflect more than grants, contracts, and dollars; they led to meaningful results – effective diagnostic tests, treatments, and vaccines – within a remarkably short time.



0 Comments