
Guest post by Anna M. Fine, PharmD, MS, Acting Director of ClinicalTrials.gov at the National Library of Medicine (NLM), National Institutes of Health. Originally posted on the Musings from the Mezzanine blog.
ClinicalTrials.gov is the world’s largest database of privately and publicly funded clinical trials. It provides easy access to clinical trial information for millions of users every month—from patients and their advocates to data submitters, data researchers, and the broader public. NLM is in the midst of a multi-year effort to modernize ClinicalTrials.gov to deliver an improved user experience on an updated platform that will accommodate future growth and enhance efficiency.
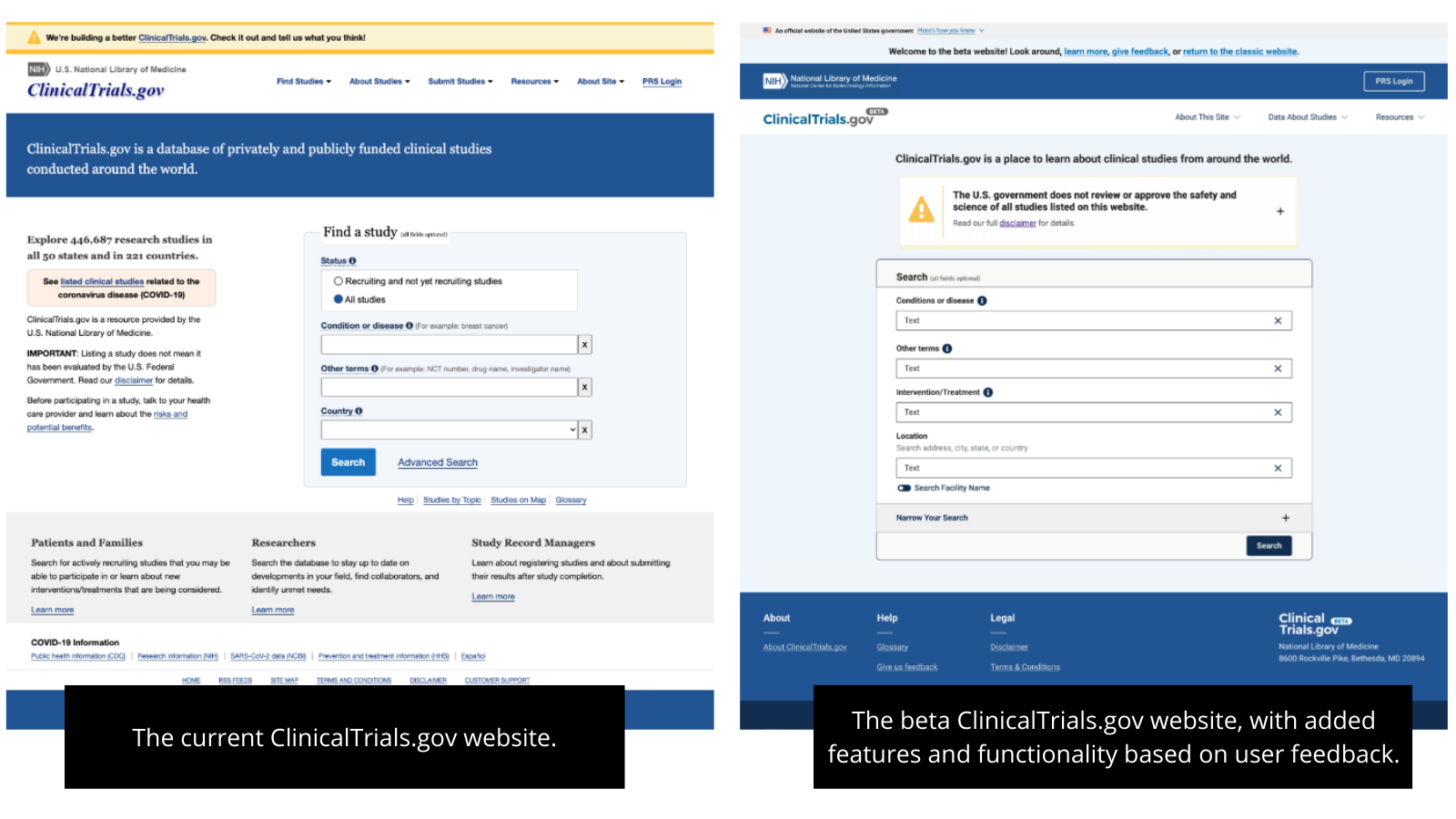
In June 2023, we will reach an important milestone: replacing the current website with the modernized ClinicalTrials.gov website. This modernized site will implement the innovations we have designed based on user feedback, including an updated look and feel and improved functionality for searching, viewing, and downloading information about clinical trials.
In advance of this launch, NLM will host a virtual public meeting on April 25 to provide a detailed look at the modernized website, a summary of our overall progress, and a look ahead to the final phases of and expectations for the modernization effort. Attendees will have the opportunity to hear from and interact (both via chat and during two simultaneous breakout sessions) with the ClinicalTrials.gov modernization team members and other stakeholders. For more information about this public meeting, please visit the ClinicalTrials.gov Modernization webpage.
In the meantime, we wanted to share key highlights of our progress and provide insight into where we’re going.
What’s Been Done
NLM announced the launch of the beta versions of the modernized ClinicalTrials.gov website and its clinical information submission and management portal, the Protocol Registration and Results System (PRS), in December 2021. Throughout 2022 and into early 2023, we made available a series of additional beta releases with new features and enhancements based on user testing and feedback.
A closer look at some of these features is available in demonstration videos for ClinicalTrials.gov Beta and PRS Beta. We will continue to make available additional beta releases of the modernized PRS into 2024, which will be summarized in the PRS Beta Release Notes.
What’s to Come
 In addition to switching over to the new ClinicalTrials.gov in June, we will continue to make additional enhancements to the system:
In addition to switching over to the new ClinicalTrials.gov in June, we will continue to make additional enhancements to the system:
- We will continue to enhance the recently released ClinicalTrials.gov beta application programming interface (API)—a toolbox that allows users and other computer systems to reuse information (e.g., patient advocacy websites, data analyses). It will be updated regularly based on user feedback, and we will provide a migration guide in the future to make a seamless transition from the existing version of the API to the updated API.
- Later this spring, we will make available a beta release for a modernized PRS, which will include the full set of protocol registration modules. Data submitters will be able to use the beta PRS to enter actual trial registration information for submission to ClinicalTrials.gov and provide feedback. The remaining protocol registration workflow will be added in future beta releases, and work towards releasing the modernized PRS will continue into 2024.
- We will continue to make the current ClinicalTrials.gov website accessible after the launch of the modernized site. The current site will be retired in 2024—more information will be forthcoming.
All the engagement activities we conducted over the last three years to identify stakeholder needs—including the modernization request for information, user surveys, and usability research, as well as our work to iteratively develop user-centered designs—will come together in these final years of the effort. Beyond 2025, we will continue to engage with users across stakeholder groups, with the aim of helping everyone reap the full benefits of the modernized ClinicalTrials.gov website and PRS.
We Want to Hear from You!
Because the ultimate success of the modernization effort is best measured through feedback, all stakeholders are encouraged to become involved by sharing their comments and suggestions via the feedback buttons on the ClinicalTrials.gov Beta and PRS Beta sites.
For additional information about modernization-related events and resources, visit the ClinicalTrials.gov Modernization webpage.
We thank you for your patience as we work to provide these updated features and improved functionality to serve our millions of users around the world. We hope you’ll join us on April 25 to hear directly from the modernization team members and stakeholder representatives who are behind this substantial effort.



0 Comments