1 Comments
Good news for trainees and principal investigators who work on training grants! eRA is readying a redesigned xTrain module, scheduled to be rolled out on March 30. xTrain is used by program directors/principal investigators (PD/PIs), university administrators, and trainees to electronically prepare and submit PHS 2271 Statement of Appointment Forms and PHS 416-7 Termination Notices for institutional research training grants, institutional career development awards, individual fellowships, and research education awards.
eRA collaborated closely with an external user group on the xTrain redesign. The new xTrain is more task-oriented, giving users streamlined workflows for their most frequent tasks. Screens are also tailored for each specific role, giving users just the information they need, when they need it.
New Visual Appearance
As part of the redesign, xTrain will adopt the new visual appearance of other eRA modules. See Figure 1 for a sample of how the new screens will look.
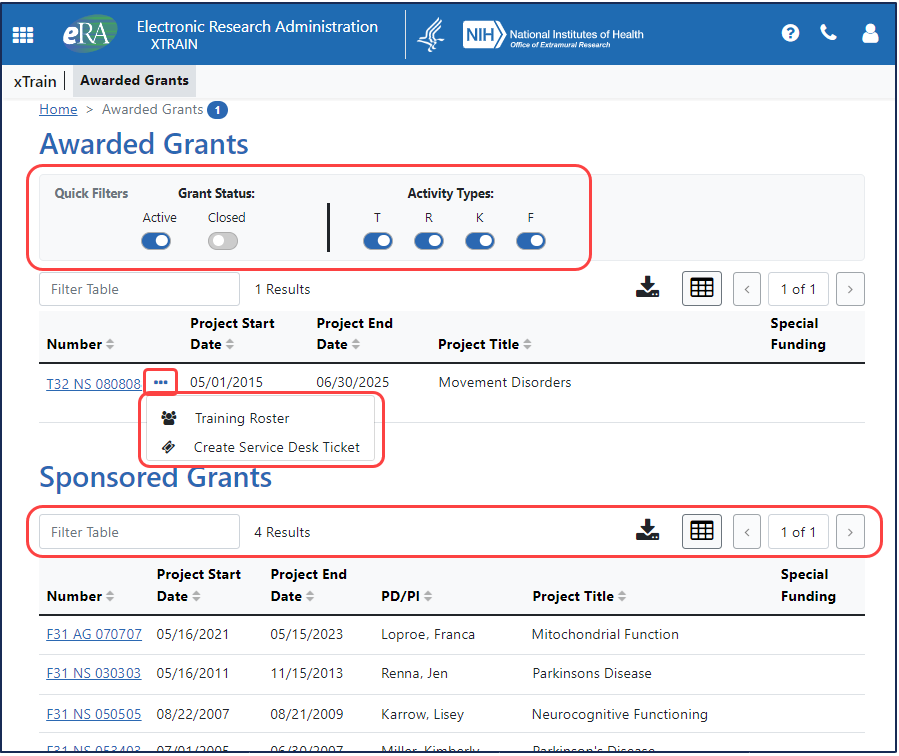
New Productivity Tools
Looking for a particular grant type? A series of toggle filters lets users quickly narrow their search for specific grants — Training (T) grants; Research (R) grants; Career (K) grants; and Fellowships (F).
Need help from the Service Desk? Users can create a Service Desk ticket from within the module by clicking a three-dot ellipsis menu in xTrain; data is prefilled from the current user’s account .
Looking to narrow a search? New table tools throughout help users improve productivity. Looking for a T32? Type it in the Filter Table field the search results are immediately filtered, showing only those records that have matching text.
Want to print or download data? Users can also download or print records and choose how many records they would like to appear on each page of results.
See Figure 1 above for examples of these tools.
A Clarification on Signatures for Trainees; on Organizational Responsibilities for External Users; and on Deletion of Forms
The release will also accommodate a clarification on signatures for trainees and a clarification of responsibilities for organizations, which are summarized below and detailed in NIH Guide Notice NOT-OD-23-094.
Trainees will be required to sign Reappointments and Amendments, just as they do for Initial Appointments, unless the trainee is unavailable, in which case the program director/principal investigator will add a comment to explain why the trainee could not sign.
The guide notice referenced above clarifies an organization’s responsibilities for Change of Recipient Organizations (Type 7) and Successor-in-Interest/Name Change (Type 6) actions.
The process for deletion of forms will change. Agency staff will no longer be able to delete forms in xTrain submitted to NIH by the recipient organization; instead, agency staff will return the form to the organization to delete.
To get familiar with the redesigned xTrain, check out the xTrain Overview video. For complete screenshots and steps, see the xTrain online help, following the March 30 release.



Thank you for the update. I would like to inquire as to the anticipated implementation of the childcare allowance tracking within xTrain (as per the NIH Notice NOT-OD-21-177 (Dated 9/23/2021). Please advise and thank you.