1 Comments
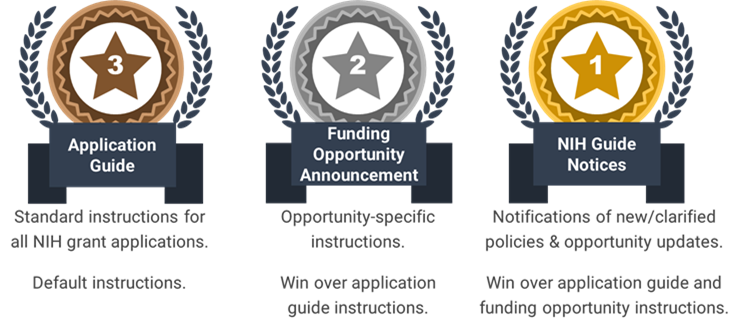
NIH grant application instructions can be found in several places:
- How to Apply – Application Guide (often simply referred to as Application Guide)
- Funding Opportunity Announcements (FOAs), and
- Notices posted in the NIH Guide for Grants and Contracts.
It is important to read and follow all provided guidance. What happens when guidance between these sources conflicts? Which one wins?
In summary, NIH Guide Notices win over application guide and funding opportunity announcements when instructions conflict. Stay up to date and subscribe to the NIH Guide for Grants and Contracts!



Great topic!
What about a conflict between the Grants Policy Statement and the Research Terms & Conditions? For example, the RTC prior approval matrix states that prior approval is required for a “change in a key person specified in the application or Federal award” but the NIH GPS states that “The requirement to obtain NIH prior approval for a change in status pertains only to those personnel NIH designates in the NoA regardless of whether the applicant organization designates others as senior/key personnel for its own purposes.”
Though the current GPS was issued/updated after the prior approval matrix, this language existed in previous GPSs as well. One of the primary purposes of the RTC (and the prior approval matrix in particular) is to serve as a quick reference guide that reduces the need to search through agency policy statements for these types of questions. If NIH is waiving this requirement, why isn’t it listed as waived in the matrix?
Thank you!