You likely know that for human-participant research funded wholly or in part by NIH, we automatically issue Certificates of Confidentiality (CoCs) as a term and condition of award. CoCs protect identifiable, sensitive information of people who participate from being disclosed to others not associated with the study. More about the CoC policy can be found on this blog and podcast. But, for human-participant research funded by an entity other than NIH, did you know that you can reach out to us to request a CoC as well?
We offer this service for certain non-NIH funded projects to strengthen the privacy protections afforded to people involved in research studies. As with all CoCs, the protections for data or biospecimens collected under an active CoC issued for a non-NIH funded project also continue in perpetuity.
To obtain a CoC for a non-NIH funded project, investigators may request one through the eRA CoC System. NIH will ensure the research meets a number of requirements, including ensuring that the activity is actually research, that it falls within the mission of NIH or HHS, and that at least one institution or performance site is within the U.S or some of the data will be maintained within the U.S. On average, it takes about two days to process requests, though some can take longer. See our FAQs on Certificates for Non-NIH, Federally Funded Research and Certificates for Non-Federally Funded Research to learn more.
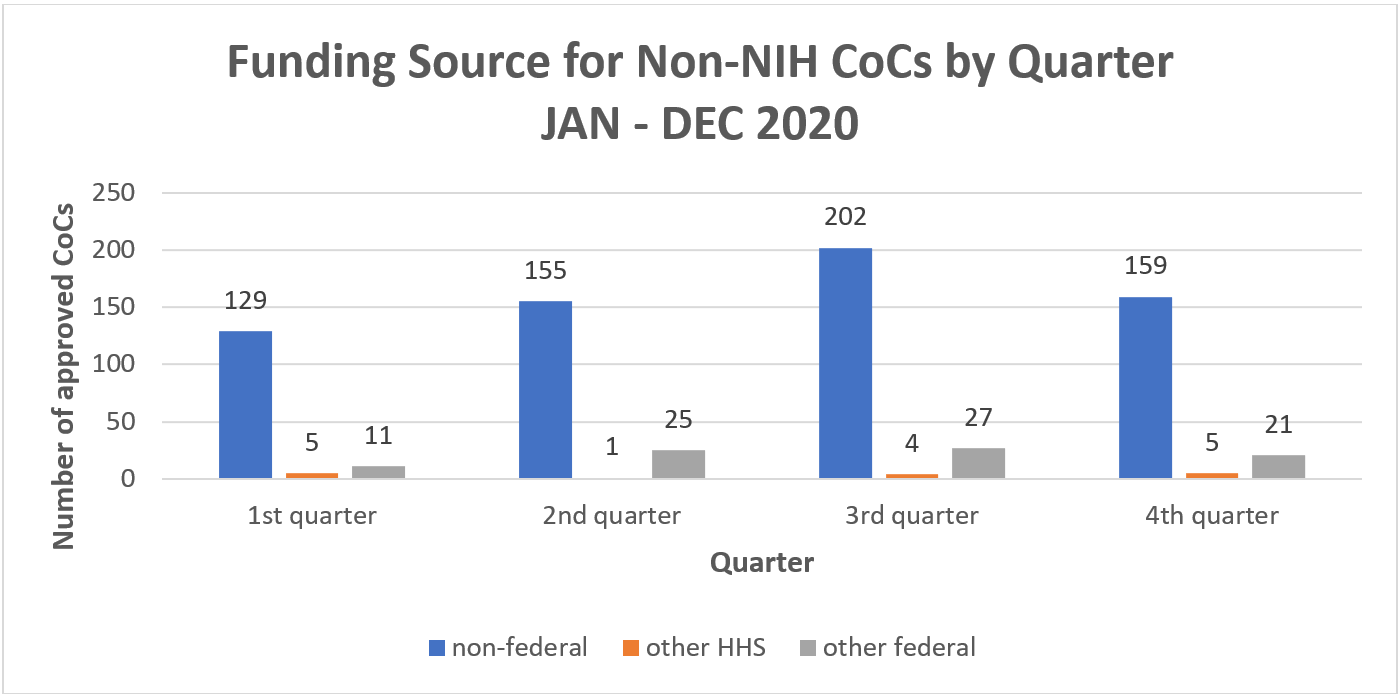
CoC requests come in for projects supported by many different funders. Non-federal sponsors, like research institutions, non-profit organizations, and pharmaceutical companies, consistently represent the bulk of approved requests. Figure 1 shows a breakdown by quarter last year. The remainder of requests issued come from human-participant research projects supported by other HHS funding sources, as well as the Department of Defense, Department of Veterans Affairs, the National Science Foundation, and other federal departments.
We have seen a steady increase in CoCs issued for non-NIH funded research over the last five years (Figure 2). Last year, NIH approved 744 CoCs, an 85% increase over 2016.
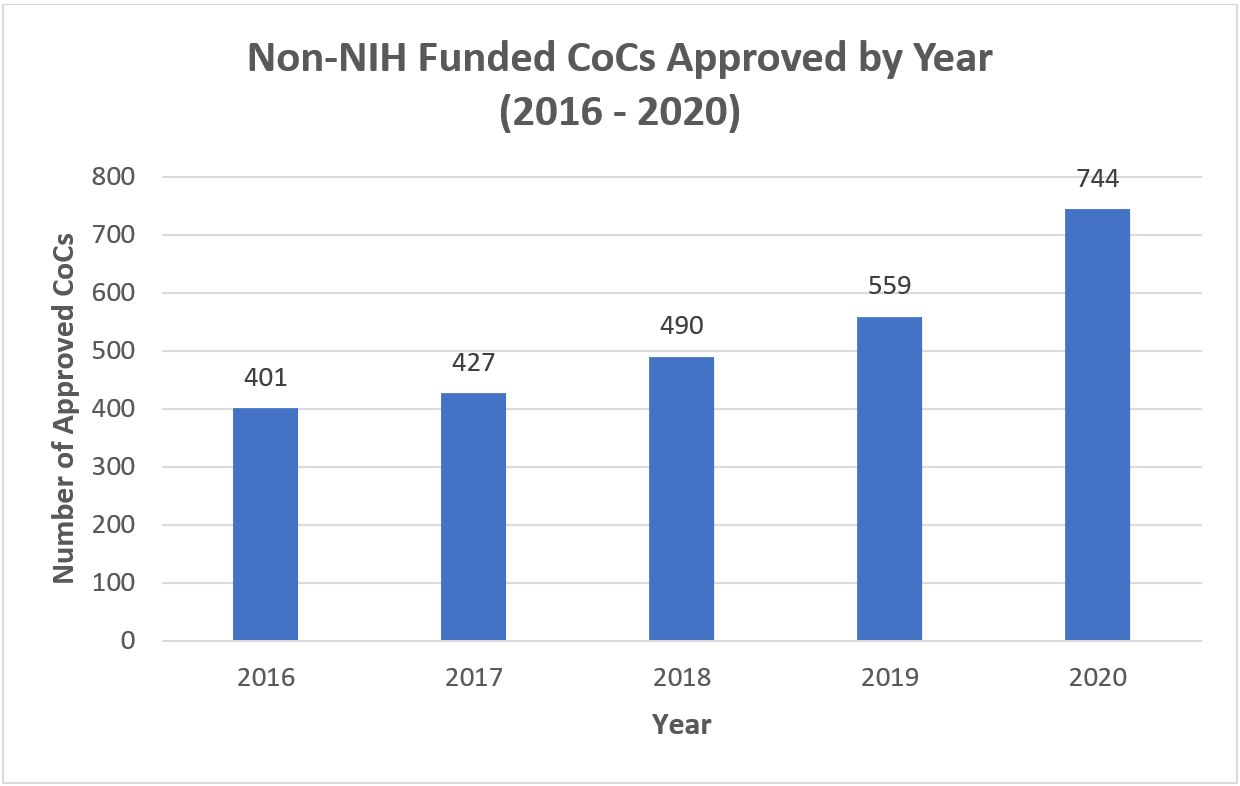
The growth of CoCs requested and approved continuing into 2020 was surprising. We expected the number of CoCs issued would decrease with pandemic shutdowns. The 2020 rate was more than what was seen for previous years, even when excluding the 126 CoCs approved for COVID-19 related research studies (16.2 percent of the total).
The delays resulting from pandemic shutdowns could extend a project beyond the approved CoC expiration date. This could cause investigators to spend additional time and resources reapplying for new Certificates and put participants’ privacy at risk. Therefore, NIH informed the 1,356 active CoC holders who found themselves in this situation that they were issued an automatic one-year extension on the expiration date (see this FAQ for more). To prevent this issue going forward, NIH removed the language about an Expiration date on CoCs issued on or after January 12, 2021 to non-NIH funded projects.
We are proud to offer this service to the biomedical research community. If you are interested to learn more, please review our CoC webpage and email any questions to [email protected].
I would like to thank my colleagues with OER’s Division of Human Subjects’ Research for their work on this service.



0 Comments