Guest post by Rebecca Williams, PharmD, MPH, acting director of ClinicalTrials.gov at the National Library of Medicine, National Institutes of Health.
As ClinicalTrials.gov celebrates its 20th anniversary on February 29, 2020, we’re asking for your input on how it can best continue to serve your needs for many more years to come.
ClinicalTrials.gov is the world’s largest public clinical research registry and results database, giving patients, families, health care providers, researchers, and others easy access to information on clinical studies relating to a wide range of diseases and conditions. This online resource, which has more than 145,000 unique visitors every day, is operated by NLM and makes available information provided directly by the sponsors and investigators conducting the research.
NLM has launched an effort to modernize ClinicalTrials.gov to deliver an improved user experience on an updated platform that will accommodate growth and enhance efficiency. Creating a roadmap for modernization requires feedback from a wide array of stakeholders on how to continue serving, balancing, and prioritizing their varied information needs. These stakeholders include sponsors and investigators who submit clinical trial information to the site, academic institutions, nonprofit and advocacy organizations, government agencies, and the public, all of whom can access and use the information that ClinicalTrials.gov contains free of charge.
To obtain timely, detailed, and actionable input, we have issued a Request for Information (RFI) to solicit comments on the following topics: website functionality, information submission processes, and use of data standards.
Recognizing that ClinicalTrials.gov supports a network of stakeholders who contribute to, and rely on, clinical research, our aim is to understand how the system can better support this network and to identify opportunities for improving its compatibility with existing clinical trial management tools and processes. It is important to note that this RFI focuses on the functionality of ClinicalTrials.gov and is not intended to modify existing legal and policy requirements for clinical trial registration and results submission.
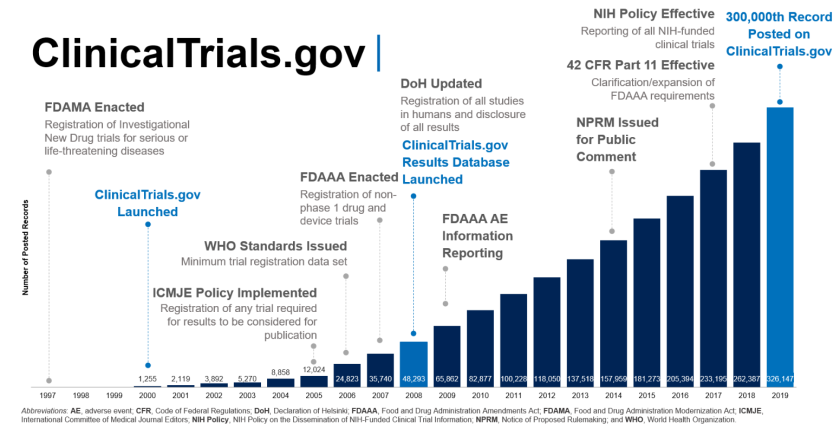
Over its 20-year history, ClinicalTrials.gov has helped shape the way in which clinical trial information is made transparent and discoverable to the public (see figure 1). In 2000, sponsors and investigators began submitting structured summaries of clinical trial protocols for the public to view. Over time, new policies and laws reinforced this practice, and ClinicalTrials.gov now contains over 320,000 study listings, with 56,000 studies currently seeking participants.
In 2008, ClinicalTrials.gov added its results database for sponsors and investigators to share summary results after trial completion. There are now over 40,000 results summaries posted on ClinicalTrials.gov, providing the public with timely access to information that may not be available in the peer-reviewed literature.
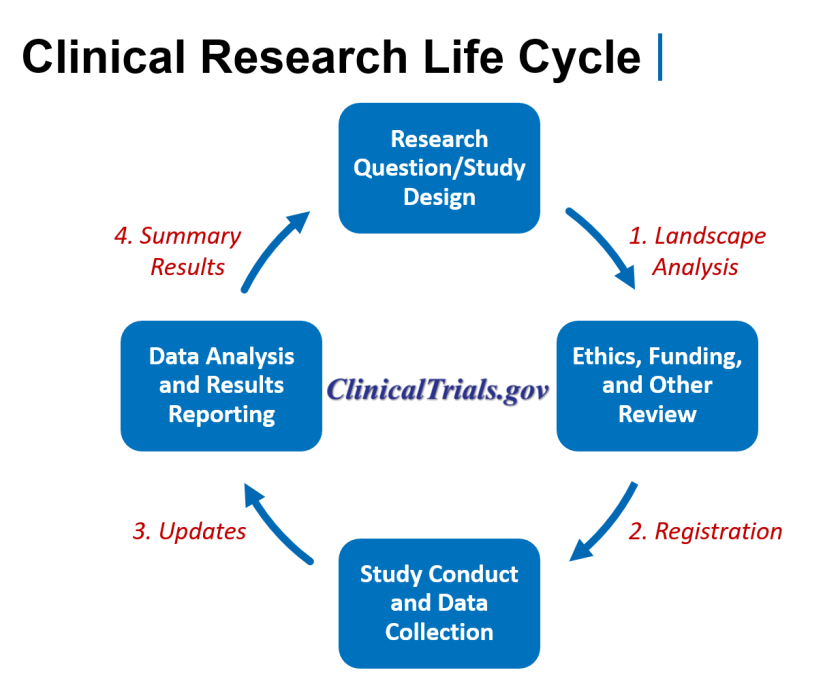
Sharing information throughout the life cycle of a clinical trial (see figure 2) supports conduct of a landscape analysis prior to conducting new research and advances important public health goals, including supporting people who are looking to participate in clinical research, tracking the progress of clinical trials, allowing for the evaluation of the integrity of reported research, and providing more complete clinical trial information to help inform patient care. The modernization effort currently underway builds on the solid foundation established during the last 20 years.
Share Your Feedback!
Responses to the RFI must be received by March 14, 2020. We expect a wide range of comments and are taking steps to manage and share the feedback. We will summarize the responses during a public meeting on April 30 on the main campus of the National Institutes of Health in Bethesda, Maryland, that will also be accessible by webcast. Details on the meeting will be available soon. In addition, we are engaging the NLM Board of Regents to provide input as we develop a roadmap for modernization, including establishing priorities and identifying the roles that various stakeholders might play in modernizing ClinicalTrials.gov.
Want to Learn More?
To learn more about the RFI and how to share your feedback, please join us for a webinar on January 22. We look forward to working with you to learn more about — and consider how to meet — your needs as we embark on this multiyear modernization effort.

Rebecca Williams, PharmD, MPH, oversees the ClinicalTrials.gov program. Her research interests involve improving the quality of reporting of clinical research and evaluating the clinical research enterprise.



0 Comments