1 Comments

Last December, NIH announced a revision to its Inclusion of Children Policy to expand the policy to individuals of all ages (NOT-OD-18-116). The revised policy, now called the Inclusion Across the Lifespan policy, requires individuals of all ages (including children and older adults) be included in clinical research studies unless there are scientific or ethical reasons to exclude them (see also this Open Mike post and this All About Grants podcast).
A recent Viewpoint Essay published in the Journal of the American Medical Association (JAMA), co-authored by Drs. Marie Bernard (National Institute on Aging), Janine Clayton (NIH Office of Research on Women’s Health), and Michael Lauer, highlights the need for such a policy. The essay summarizes efforts by NIH to implement 21st Century Cures requirements to publish data on the age of research participants, to convene a workshop on age grouping and exclusions, and to make a determination on whether to revise inclusion guidelines on age.
A common theme that emerged during implementation of these 21st Century Cures requirements was the need for more data on the age of participants in NIH clinical research. Participants at NIH’s June 2017 Inclusion Across the Lifespan Workshop identified lack of easily accessible, detailed information on the number of children and older adults as a barrier to understanding study outcomes across age groups. Respondents to a Request For Information on the topic requested age-related individual-level data be provided for each NIH-funded study using existing mechanisms. We also heard stakeholder feedback about the need for flexibility in age groupings, as meaningful units of measurement may vary depending on the population under study.
Until now, NIH has not systematically collected data on the age of participants enrolled in the research it funds. The limited information about the age of participants in our research leaves us unable to answer questions such as, “what percentage of NIH-funded projects investigating epilepsy involve toddlers?” But soon, we will be a step closer to answering such questions.
For applications submitted for due dates January 25, 2019 or later, NIH will require awardees to submit de-identified participant-level data on sex/gender, race, ethnicity, and age at enrollment in progress reports. These data will be submitted in .csv format and specify the sex/gender, race, ethnicity, and age at enrollment of participants, in units from hours to years (Figure 1). Instead of aggregating participant demographic data, investigators can skip that step and let our system do the aggregating for them. By collecting participant data in this way, NIH expects to maximize flexibility and detail for analyses of our portfolio across the entire lifespan, while minimizing burden on the investigator.
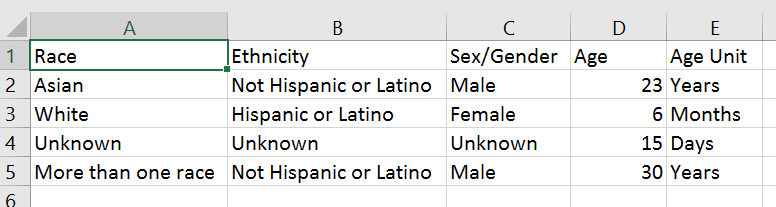
Figure 1
With these data in hand, for the first time, NIH will be able to provide detailed data on the age of participants in NIH-supported clinical research studies. NIH expects this policy to bring us a step closer to understanding the representation of various age groups in NIH-funded research and to help us identify opportunities across the research portfolio related to age-based inclusion.
NIH is committed to supporting clinical research that benefits individuals of all sexes/genders, races, ethnicities, and ages. We look forward to the availability of additional data to help us better understand the distribution of participants in our clinical research, and ultimately how interventions work in the populations to which they will eventually be applied.



Is there an image anywhere that shows what the input form and finished form for this reporting will look like?
Thanks,
Lisa