
 At NIH, we maintain a broad and diverse portfolio of biomedical and behavioral research. To turn discovery into health, promising technologies must move from the laboratory into clinical trials, into the marketplace, into the doctor’s office, and into our every-day lives. A key way to transition promising technologies out of the laboratory is through the commercialization process.
At NIH, we maintain a broad and diverse portfolio of biomedical and behavioral research. To turn discovery into health, promising technologies must move from the laboratory into clinical trials, into the marketplace, into the doctor’s office, and into our every-day lives. A key way to transition promising technologies out of the laboratory is through the commercialization process.
Small businesses help to achieve this commercialization goal. By strategically aligning research resources in academic settings with the dedicated funding for small businesses that Congress established decades ago, NIH can reduce the barriers to entry and help entrepreneurial scientists move their promising products through the development pipeline. Here, and in subsequent upcoming posts, we will highlight some programs at NIH that can help you chart a course through the commercialization process.
Sailing on the Flagship SBIR/STTR…
Let’s start by discussing the largest source of early-stage capital for technology commercialization in the United States. Known as “America’s Seed Fund,” the flagship Small Business Innovation Research (SBIR) and Small Business Technology Transfer Research (STTR) programs at federal funding agencies are critical to support feasibility testing, proof-of-concept, and research and development of commercial products—this goes for NIH too. These awards are made to U.S.-owned and operated for-profit institutions such as existing small businesses, start-up companies, or spin-offs from academic labs, to move their ideas through to commercialization.
SBIR awards support small businesses to develop and commercialize innovative technologies. STTR awards support collaborative opportunities for small businesses and nonprofit research institutions, providing a bridge to develop academic discoveries within a small business context for commercialization of resulting innovations.
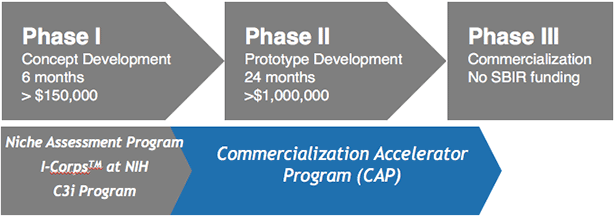
The SBIR/STTR programs consist of three phases, providing funding for proof of concept/feasibility testing during Phase 1 and additional funding for full R&D/prototype development during Phase 2. SBIR/STTR funds do not, however, support Phase 3, the actual commercialization of the technology, but this commercialization phase may receive support from other sources of Federal funds.
Statute determines how much federal agencies spend on their SBIR/STTR programs. Federal R&D funding agencies are required to support SBIR activities if their extramural research budget is more than $100 million, and STTR activities if that budget exceeds $1 billion. In fiscal year (FY) 2017, NIH’s share of its extramural research budget set-aside for SBIR/STTR awards was raised to 3.65 percent, from 2.8 percent in FY 2010, which aimed to help advance biomedical technologies through the regulatory review process and on to the market.
NIH is the second largest small business funder across the government. In fiscal year 2017, over $980 million in SBIR and STTR projects were awarded to over 1,300 health and life science companies. Even with this robust financial support, innovators still face significant knowledge and resource barriers when trying to enter and grow within the biomedical commercial space. After all, it takes more than SBIR/STTR funds alone to develop a technology. SBIR/STTR investments are meant to catalyze product development to a certain point, where small businesses would then next raise additional funds from private sources.
Being on an Even Keel…
Recognizing the challenges to bringing innovative technologies to the market, NIH has developed diverse approaches to help investigators traverse these barriers. Adapting best practices from the National Science Foundation for example, we established the eight-week I-Corps at NIHTM program. Investigators who participate in this program receive mentorship from biotech sector experts, networking opportunities, and guidance to build a comprehensive business model. The iinitial I-Corps cohorts at the NIH have raised over $60 million in strategic partnerships and investments since 2014, spun-off additional companies and have had one company undergo a successful merger/acquisition.
Wondering where the business opportunities are? Well, NIH resources are helping to address this too. Various teams of medical device innovators are now able to access specialized business tools, curricula, and mentorship to better understand their technology’s commercial value and business opportunity. Companies should look at the Concept to Clinic: Commercializing Innovation (C3i) program at NIBIB as an example. As a testament to this initiative, 38 teams thus far have received over $12 million in NIH SBIR/STTR supplement awards and raised over $32 Million in private equity financing.
Navigating through the Currents…
Sometimes, without the same administrative support structure at larger institutions, small businesses can lose their way and not complete the SBIR/STTR application process. Therefore, we are piloting the NIH Applicant Assistance Program (AAP) to help new and/or previously unsuccessful small businesses submit a competitive application to funding Institutes or Centers, like NCI, NHLBI, and NINDS.
Moreover, NIH-supported innovators, even with the most promising technology, may have trouble navigating regulatory approvals. We at NIH are seeking to demystify this regulatory process and provide as much support for applicants as possible. NHLBI recently issued a funding opportunity announcement aimed at providing regulatory support allowing SBIR/STTR awardees to further develop their medical devices. Small businesses, who were awarded an administrative supplement through this initiative, traveled to FDA to demonstrate their innovation and receive face-to-face mentorship. These companies gained critical insights and strategies early in the regulatory process, clinical trial organizational know-how, and validation of their development plans—critically important for first-in-human testing in the United States. To further build on their desire to help small businesses, NHLBI also created this handy video resource on how to navigate FDA, and other information on intellectual property, commercialization plans, and reimbursement. Strong partnerships like this with FDA demonstrate our joint commitment to ensuring pivotal new medical devices have a better chance to be available sooner in the US.
More Beacons in the Night…
At no charge, NIH provides access to additional programs that facilitate a company’s success. The Niche Assessment Program provides market insights and data to help small businesses with SBIR/STTR Phase I awards strategically position their biotechnology in the market. By gaining insights into developing commercialization plans for a Phase II SBIR/STTR application, participants learn about customer needs, competing products, pricing, industry trends, and barriers to market entry.
The 9-month mentoring Commercialization Accelerator Program (CAP) provides Phase II awardees with a combination of deep domain expertise and access to industry connections. Having gained insights into establishing market relevance, building commercial relationships, and focusing on revenue opportunities as part of this program, participating companies have parlayed their new industry experiences to achieve measurable gains and accomplishments. The nearly 1,000 companies that have taken advantage of this initiative since 2004 have raised over $1 billion in additional financing, had 44 acquisitions, and have had 5 initial public offerings.
Steering Towards the Lighthouse…
We are taking many diverse approaches to build on our small business programs. We recognize that a robust innovation ecosystem made up of basic laboratory discoveries, pre-clinical testing, assessment in human trials, and commercialization are all critical to translate scientific findings into improved public health. The resources presented here are just the tip of the iceberg. Please stay tuned for more information on helping small businesses get established, successfully compete for SBIR/STTR awards, and maximizing interactions with other investors.
Interested in learning more? Success stories (such as available here and here) and other relevant information can be found on our small business website. Please also consider joining us at the annual HHS SBIR/STTR conference or another small business events, where you can learn about various programs and funding opportunities, writing successful SBIR/STTR grant applications, and much more.



0 Comments