In the rapidly evolving world of modern medicine, it is important that the transition of basic scientific discoveries into new medical treatments takes place with both precision and speed.
NIH’s Small Business Innovative Research (SBIR) and Small Business Technology Transfer (STTR) programs – which I’ve discussed a few times on the blog before – are a key part of NIH’s translational research portfolio.
Each year, the SBIR and STTR programs provide over $750 million dollars to small US-based companies, often with deep roots and collaborations with research institutions, to conduct early stage innovative biomedical research with commercial potential. SBIR and STTR funding helps validate cutting-edge ideas through milestone-driven animal and early clinical studies or prototype development. The programs also help small businesses attract private capital and industry partnerships often needed to cross what’s commonly referred to as “the valley of death.”
However, achieving technological feasibility is only one aspect along the path of commercialization. Academically trained principal investigators do not necessarily have the experience or business acumen to run a company. But that’s okay – NIH has several important resources to help them, one of which just celebrated its 10 year anniversary.
The NIH SBIR/STTR Commercialization Assistance Program (CAP) is a 9-month mentoring and training program, designed to help NIH SBIR/STTR Phase II grantees develop technology-specific commercialization strategies, such as penetrating crowded markets, successfully negotiating with investors, or navigating the FDA regulatory process. With a notoriously high attrition rate of biotech firms, CAP is widely recognized for enabling small businesses to grow and succeed.
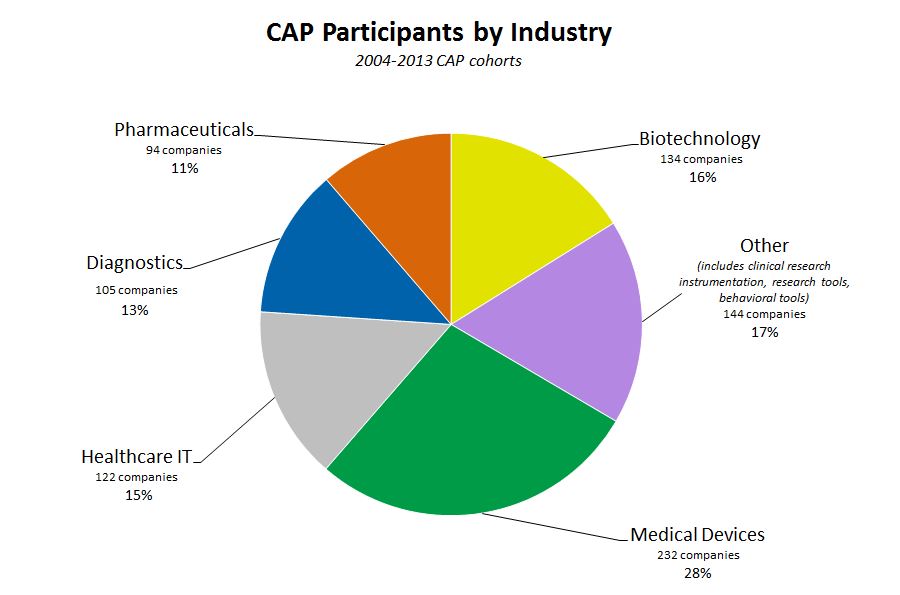
As the pie chart below demonstrates, CAP works with SBIR/STTR awardees across the research spectrum from biotechnology, diagnostics, healthcare IT, medical devices, and pharmaceuticals to research tools and behavioral modification applications.
 CAP enlists the help of industry experts with current working knowledge of their domain areas and understanding of current market and regulatory trends, then connects them with SBIR and STTR grantees to help them with the many facets of developing their business or technology. This can include: evaluating their prospects for third-party investments; strategic partnerships or licensing opportunities; helping them articulate a compelling business case for their innovation; and providing helpful networking opportunities.
CAP enlists the help of industry experts with current working knowledge of their domain areas and understanding of current market and regulatory trends, then connects them with SBIR and STTR grantees to help them with the many facets of developing their business or technology. This can include: evaluating their prospects for third-party investments; strategic partnerships or licensing opportunities; helping them articulate a compelling business case for their innovation; and providing helpful networking opportunities.
Over the past 10 years, CAP has trained over 830 companies and approximately 70% of participant companies reported that the NIH CAP had a major or valuable impact on their commercialization progress. From 2004 – 2013, the NIH CAP has also helped small businesses raise over $586,000,000 in non-governmental funding; sign over 586 deals, including 26 acquisitions, distribution agreements, licensing agreements, and investments; create over 1,680 jobs; and form thousands of strategic partnerships from the vast CAP network of industry experts and advisors.
We invite you to learn more about this important program at NIH by visiting the newly redesigned NIH SBIR/STTR website, visiting the CAP homepage, and viewing the success stories from this program. If you have any questions about the NIH SBIR/STTR programs, get in touch with the NIH SBIR office, and be sure to check out ways you can stay connected with these programs.



0 Comments