It depends. Only BESH studies that were awarded through a BESH-specific funding opportunity are eligible for registration and reporting flexibilities (as noted in NOT-OD-21-088). BESH studies that came in through funding opportunities designated as ‘Clinical Trial Required’ or ‘Clinical Trial Optional’ must register and report results in ClinicalTrials.gov according to the Clinical Trial Dissemination Policy.
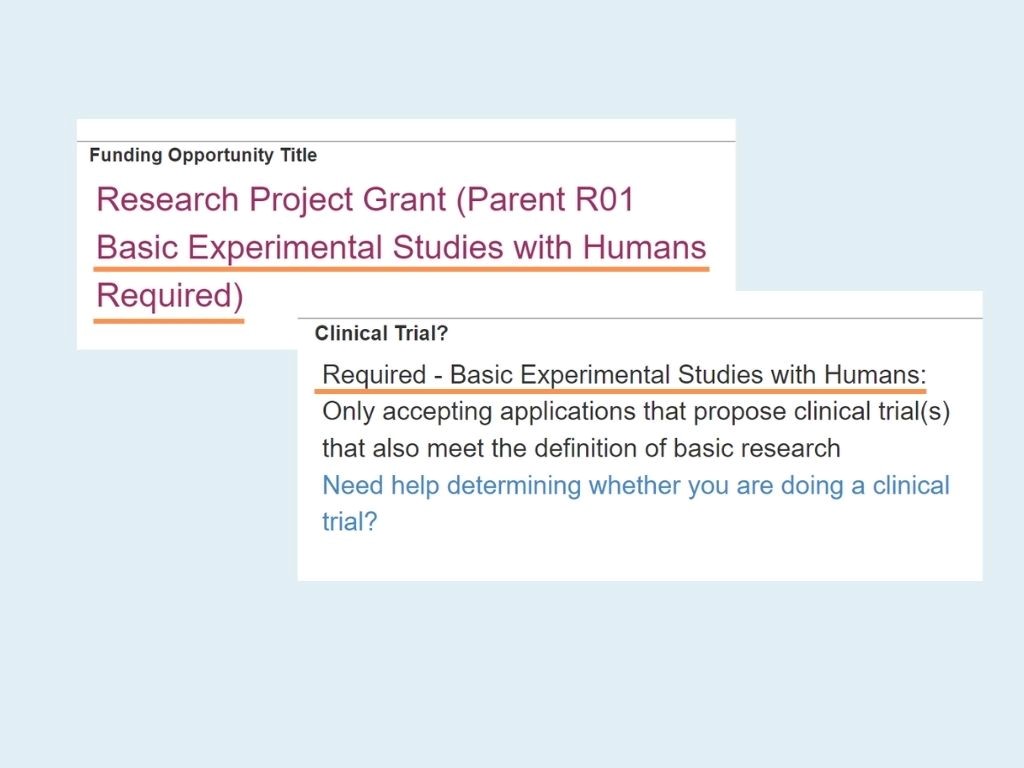
Not sure if your FOA is designated as BESH? All FOAs have a clinical trial designation listed in the Title and in the body of the announcement in Section II, as illustrated below.
For more resources and information, see our webpage on Basic Experimental Studies involving Humans and this podcast episode.



0 Comments